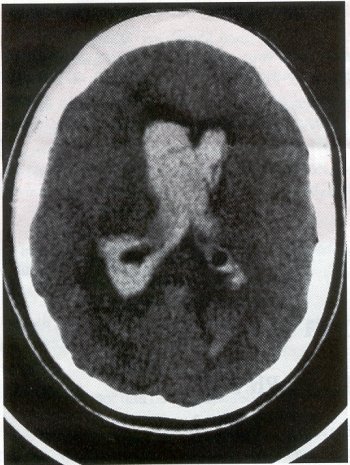
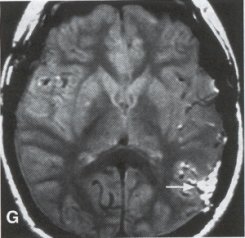
| Moyamoya disease is a progressive disease of the distal internal carotid arteries and their major branches that is characterized by occlusion of these vessels, which show intimal proliferation. A characteristic finding is the formation of a collateral network of blood vessels at the base of the brain with an unusual angiographic appearance described as a "puff of smoke." This disease was first reported by Takeuchi and Shimizu in 1957, and numerous other cases have been reported since then. Kudo introduced the disease to the English literature in 1968, and the term moyamoya disease was first used in 1969. Moyamoya disease is more common in Asian populations, but even in Japan the overall incidence remains below 1 per 100,000. The male-to-female ratio has been shown to be 1:1.65 in one large series, but other series have included too few patients to determine whether one sex is more affected. The peak age of onset of moyamoya disease in the Asian population is bimodal, with an early peak occurring in the first decade of life, and a second peak in the fourth decade of life. In one Asian series of moyamoya patients, the mean age of diagnosis was 5 years for the early peak and 36 years for the later peak. Moyamoya commonly involves both intracranial hemispheres, but unilateral disease has been reported. Children afflicted with moyamoya disease usually present with strokes; transient ischemic attacks, which may alternate sides; slowly progressive cognitive decline; seizures; or involuntary movements of the extremities. Many of these episodes can be induced by crying, coughing, or straining. Adults are believed to present more commonly with intracranial hemorrhage (Fig. 1), including subarachnoid hemorrhage, and less commonly with ischemic symptoms. However, in our experience with adult moyamoya patients treated at Stanford, 89% presented with ischemia, 7% with hemorrhage, and 4% with both ischemic and hemorrhagic symptoms. In 70% to 80% of patients, the hemorrhage occurs deep in the basal ganglia, thalamus, and ventricular wall. The natural history of untreated moyamoya disease is poor: the rate of major deficit or mortality over the 2 years following diagnosis is 73% in children, and the prognosis is similarly poor in adults. Moyamoya disease often is accompanied by intracranial aneurysms, possibly because of the increased blood flow and hemodynamic stress in the abnormally dilated collateral vessels. Another theory speculates that there may be a congenital arterial defect thatpredisposes to both moyamoya disease and aneurysms. The presence of aneurysms may explain the higher incidence of intracranial hemorrhage in adult patients with moyamoya disease. Arteriovenous malformations (AVMs) rarely are associated with moyamoya disease, with only a few reported cases; in those patients, however, the AVM was located in the middle cerebral artery distribution and was fed by abnormal moyamoya vessels. Pathogenesis and Pathology The pathogenesis of moyamoya disease is unknown, but several associations have been made. A history of inflammation in the head and neck region has been implicated in the development of moyamoya disease, with one series showing that approximately two thirds of patients had previous inflammatory disease. This inflammation model has been reproduced in dogs - foreign protein injected into the carotid artery led to the formation of intracranial vessels with a moyamoya pattern. Other potential causes include tuberculosis meningitis, arteriosclerosis, neurofibromatosis, irradiation, sickle cell anemia, connective tissue abnormalities, and congenital causes. A hereditary factor has been proposed, because the incidence of familial cases in Asian populations has been reported as high as 7% and because the incidence of moyamoya disease in patients with Down syndrome is high. Autopsy reports in moyamoya patients show prominent intimal thickening, which in some cases is due to lipid deposition and edema. Abnormalities in the internal elastic lamina, including thinning or duplication of this layer, also have been seen. Moyamoya vessels also showed focal fibrin deposition and marked thinning of the vascular wall, primarily within the media and adventitia. Indications for Operation Indications for surgical treatment of moyamoya disease include a combination of patients' symptoms and radiographic evaluation. Many moyamoya patients are candidates for procedures to augment blood flow if they have symptoms related to ischemia or previous hemorrhage. Progressive neurologic deficits, such as cognitive decline or progressive seizures, also are indications for bypass. Medical treatment with antiplatelet agents, anticoagulants, calcium channel blockers, or other drugs does not appear to be beneficial for patients with moyamoya disease. The risk of surgical treatment is related in part to the clinical condition and other underlying medical problems of the patient. Patients who are in poor clinical condition as a result of ischemia or hemorrhage may not be ideal operative candidates unless significant improvement occurs. However, patients with significant mass effect and deteriorating neurologic condition from a hemorrhagic clot should be considered for emergent evacuation of the hemorrhage, with revascularization reserved for a later date. Revascularization should be performed under nonemergent conditions. The lack of experience of most neurosurgeons in performing large numbers of bypass procedures also may increase the risk of surgery, particularly at centers with a low volume of moyamoya patients. Radiographic evaluations also play a role, particularly in the determination of the method of revascularization. Cerebral angiography allows identification of superficial temporal arteries (STAs) of reasonable size, indicating that a superficial temporal-to-middle cerebral artery bypass can be performed. The absence of a reasonable STA vessel may lead the surgeon to perform encephalomyosynangiosis (EMS), a procedure that involves laying the temporalis muscle over the surface of the brain and does not require the presence of the STA. In instances in which the STA is very small ( < 1 mm), as in some children, EMS and encephaloduroarteriosynangiosis (EDAS) - the anastomosing of the intact STA with its soft tissue cuff to a surgical incision of the dura - are feasible options. Computed tomographic (CT) and magnetic resonance imaging (MRI) studies are useful in visualizing some of the intracranial changes in moyamoya patients, including narrowing of vessels of the circle of Willis, areas of previous stroke, and intracerebral hemorrhage. MRl is more sensitive than CT, particularly with the use of diffusion and perfusion MR images, which also allow the identification of early ischemic areas. MRI also has allowed the visualization of moyamoya vessels themselves in some instances. Electroencephalographic (EEG) studies in moyamoya patients are distinctive, and may possibly be useful as a screening test. The classic finding is the return of high-voltage slow waves on EEG 20 to 60 seconds following the termination of hyperventilation, a finding termed the "re-build-up" phenomenon. Although the mechanism that causes this finding is unknown, this pattern has not been observed in any other disease pathologies. Hemodynamic insufficiency can be characterized by severely reduced cerebral reserve capacity. This is determined with a baseline xenon (Xe-133) CT or single photon emission computed tomography (SPECT) study followed by an acetazolamide challenge or a positron emission tomography (PET) scan. The initial xenon CT scan indicates baseline cerebral flow and identifies areas of low perfusion. Acetazolamide causes vasodilatation, and a second xenon CT scan after administration of this agent evaluates the reserve capacity of cerebral blood flow. Impaired reserve or actual "steal" phenomenon after acetazolamide suggests these brain regions are at high risk for subsequent infarct. Recent studies demonstrate that the natural history of atherosclerotic cerebrovascular patients with impaired reserve or steal is extremely poor, with a 36% chance of stroke over a 24-month mean follow-up. In anecdotal series, STA-MCA markedly improves symptoms, outcome, and cerebral blood flow (CBF) measurements for such patients who have impaired CBF reserve. Other reports demonstrate that moyamoya patients also have improved hemodynamic reserve with resolution of ischemic symptoms and good clinical outcome after revascularization. Preoperative Preparation Cerebral angiograms, including bilateral internal carotid, bilateral external carotid, and vertebral artery injections, are performed to evaluate donor vessels, collateral circulation, and moyamoya vessels. The size and location of optimal vessels to be used for the revascularization are identified. CBF studies, with and without acetazolamide, are performed preoperatively. We prefer xenon CT because it is quantitative and easy to admmister. Inraoperative EEG, somatosensory evoked potentials, and microvascular Doppler studies are useful adjuncts. Postoperative bypass patency, extent of revascularization, and regression of moyamoya vessels usually are evaluated with conventional cerebral angiography, but recent studies show that magnetic resonance angiography (MRA) may correlate with conventional angiography. Postoperative CBF studies asses improvement in baseline perfusion and hemodynamic reserve.
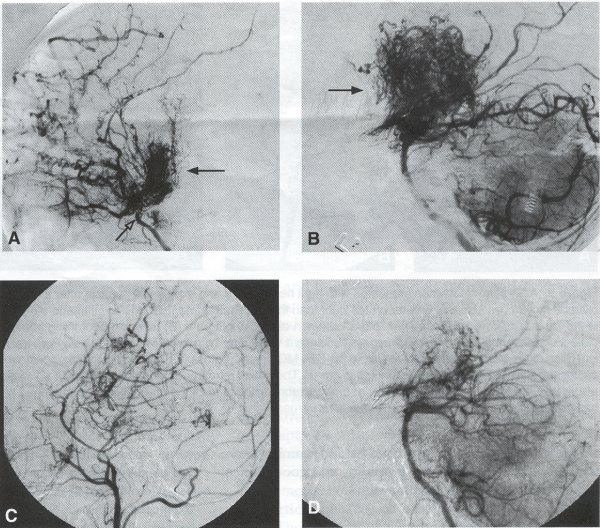
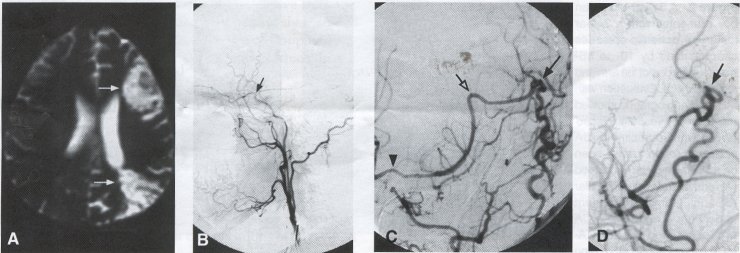
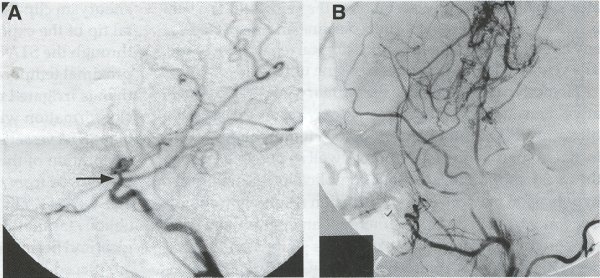
Operative Technique STA-MCA Bypass History and general surgical techniques. The first STA-MCA bypass for moyamoya disease was performed by Yasargil in 1972, and since then several small series have been reported. Karasawa et al. reported on 12 moyamoya patients under going bypass, with 10 patients (83%) having good to excellent results. Quest and Carrol reported a similar outcome in 11 of 13 patients (85%). Ishikawa et al. compared STA-MCA bypass (48 procedures) with indirect revascularization methods (16 procedures), such as encephaloduroarteriomyosynangiosis, in pediatric moyamoya patients and showed that the incidence of postoperative ischemic events was significantly reduced in the direct bypass group (10%) compared to the indirect group (56%, p < 0.01 ). They concluded that direct revascularization is the procedure of choice over indirect revascularization whenever possible. Matsushima et al. have shown a similar advantage of direct revascularization over indirect methods, although with a smaller group of patients. Since 1991 (as of May 2000), we have performed 60 STA-MCA grafts (Figs. 2, 3, 4) in moyamoya patients ages 5 to 63 years, with excellent clinical results overall. Six patients had temporary minor worsening postoperatively, which resolved with volume expansion, and one patient had a postoperative hemorrhage into a previously ischemic region. Mean follow-up was 43 months (range, 1 month to 7 years), and 95% of patients were neurologically stable or improved. One patient developed persistent TIAs after occlusion of a STA-MCA bypass with short vein interposition. Follow-up angiography showed that 97% of grafts were patent, and there was improved hemodynamic reserve on cerebral blood flow studies in most patients. There were no perioperative deaths, but one adult died of a myocardial infarction 72 months after bypass, and another adult died 18 months following an external carotid to MCA bypass using a vein interposition (from hemorrhage into ischemic brain). Of 13 pediatric moyamoya patients (21 hemispheres) treated with STA-MCA bypasses (9 following strokes, and 6 with refractory TIAs), none have developed new strokes (mean follow-up 45 months, range 2-84 months). Postoperative angiograms showed 100% graft patency and successful revascularization of the MCA distribution in over 90% of the cases, and postoperative xenon CT scans showed increased augmentation with acetazolamide as compared to the preoperative study. Several operative generalities are applicable to STA-MCA revascularization procedures. Patients are positioned with the head above the heart to reduce venous cerebral congestion. Hyperventilation and alpha-adrenergic agents are not recommended because of their vasoconstrictive effects, but mild hypothermia (32-34°C) and barbiturates are used routinely to provide a cerebral protective effect during occlusion times. The mean arterial pressure should be kept in the normal to high range, and patients should be volume expanded to prevent ischemic events. Vasoconstrictive agents such as epinephrine should not be used during scalp infiltration. Intraoperative monitoring with EEG and somatosensory evoked potentials provides early detection of ischemic changes, and thiopental may be administered during the MCA occlusion for the anastomosis. Microinstruments and the operating neurosurgical microscope are routinely used for revascularization procedures. Donor vessels should be selected with an outer diameter ³ 1 mm, because smaller vessels have higher occlusion rates, provide less blood flow, and are more difficult to anastomose. Vein interpositions may be used, and they provide immediate dilatation to increase blood flow to the recipient vessel. Papaverine is useful in preventing vascular spasm. If venous interposition is needed, the superficial temporal vein or saphenous vein can be used. The vein segments are meticulouslv harvested, and side branches are carefully ligated while avoiding any laceration to the vein itself. The vein is then irrigated with heparinized saline and inspected for any lacerations that would require surgical repair prior to use. The vein is stored in heparinized saline until needed. Hemodynamic control is the primary goal of postoperative management. Hypertension may result in excessive bleeding at the anastomotic site. Hemorrhages, from increased perfusion of the brain as well as possible leaks in the anastomotic site, are other complications. Conversely, hypotension may cause graft occlusion resulting in clinical ischemia. In such cases, an emergent angiogram and graft revision may be necessary. Aspirin is started on the first postoperative day. Cerebrospinal fluid (CSF) leak is a potential complication, due to the non-water-tight dural closure, but this is extremely rare. Operative Technique The patient is positioned with the head turned to the contralateral side and the temporal bone parallel to the floor and placed in three-point pin fixation. After the scalp is shaved, a handheld standard Doppler ultrasound probe and the preoperative angiograms are used to identify the location of the STA, which is then marked out with a marking pen. There usually are two main branches, the frontal and parietal STA branches; both should be marked out to allow a choice of vessel during the procedure. Following sterile preparation, an incision is made, beginning over the zygoma, with a #15 blade. The STA is identified and skeletonized using sharp dissection with a combination of scissors and a #15 blade. When necessary, a sterile Doppler probe can be used to confirm the course of the STA. Either the frontal or parietal STA branch may be used, depending on diameter and suitable length. Our preference is to use the larger diameter artery, with the exception being a frontal STA branch, which is larger but passes low over the forehead. It is desirable to leave a cuff of tissue around the artery itself. This reduces direct trauma to the artery, decreases vasospasm of the vessel, and allows the surgeon to grasp the donor segment without injuring the vessel. Smaller branches of the artery are bipolar coagulated; larger branches are isolated and tied off. The length of the artery required depends on the distance from the artery to the graft site. A craniotomy is performed in the superior, midtemporal bone overlying the Sylvian fissure, with care taken to avoid injury of the STA vessel during the bone opening. The dura is then opened, and the neurologic microscope is used to identify the recipient vessels. Optimal recipient middle cerebral artery (MCA) branches have outer luminal diameters ³1 mm. Other variables influencing the choice of recipient vessel include the orientation of the vessel and the location of the MCA branch relative to the Sylvian fissure (closer usually is better, because this allows improved backflow down to the ICA bifurcation). An M3 or M4 MCA branch is preferred. Dissection of the arachnoid layer over a 6- to 10-mm segment exposes this vessel. Tiny branches originating from this segment are coagulated and divided. A small piece of sterile plastic is placed beneath the segment of the MCA at the anastomosis site as a "high visibility" backfield. A temporary aneurysm clip is placed on the proximal STA, and the distal tip of the exposed vessel is ligated and cut. Blood flow through the STA can be tested by transiently removing the proximal temporary aneurysm clip. The lumen of the STA then is irrigated out with heparinized saline to minimize clot formation within the donor vessel. The distal portion of the STA vessel is trimmed to the appropriate length, based on location of the recipient vessel, and the soft tissue cuff is removed from the distal 3 to 5 mm as preparation for the anastomosis. The distal end of the donor vessel is cut in an oblique fashion. Small microvascular clamps then are placed on each side of the recipient vessel to prevent bleeding during the anastomosis. The recipient vessel is cut in a diamond-shaped fashion using a microscissors. Carmine indigo dye may be used to improve visualization of the vessel lumens. The anastomosis is performed under the neuromicroscope using 10-0 monofilament suture. Corner stitches are placed first, followed by the far wall and then the near wall. The intimal layer must be included with each interrupted stitch, but significant narrowing of the anastomotic site should be avoided. We prefer interrupted sutures, but a continuous suture can also be used. Temporary clips are then removed, first from the MCA branch, and then from the proximal STA. Total MCA branch occlusion times typically are 20 to 30 minutes. Significant bleeding may indicate the need for an additional stitch, whereas small amounts of bleeding may be treated with Gelfoam or Surgicel. Once the anastomosis is complete, a Doppler ultrasound may be used to test patency. We use a quantitative and directional ultrasound to asses flow both pre- and post-bypass. The dura is carefully closed to avoid compromising the STA. The bone flap also is trimmed to minimize any pressure on the donor vessel. Temporalis and scalp closure then proceeds in the usual fashion.
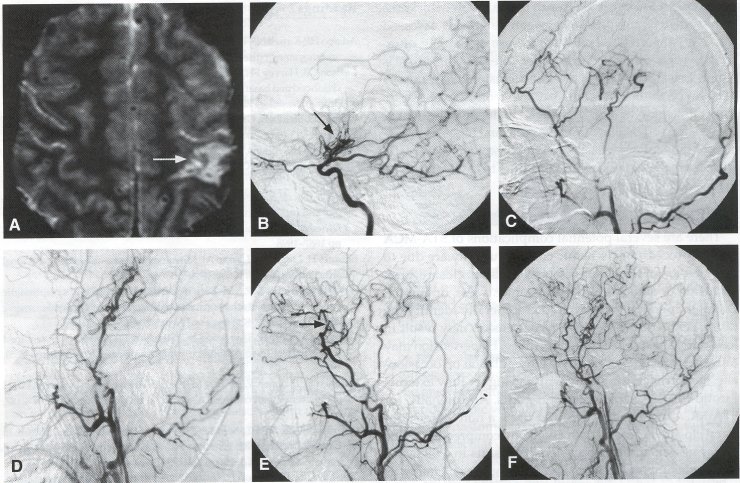
Alternative Approaches Encephaloduroarteriosynangiosis (EDAS), also known as Pial Synangiosis. On occasion, other alternatives to the STA-MCA bypass can be used to augment blood flow to the brain. In encephaloduroarteriosynangiosis, first described by Matsushima et al., the superficial temporal artery is first dissected out as for an STA-MCA bypass, but is left in continuity. The dura is then opened in a linear fashion, and the artery is placed over the exposed cortex. Initial reports indicated that the vessel could be placed over the arachnoid, but further studies show that improved collateral flow can be obtained by opening the arachnoid and allowing the STA to contact the pia. The soft tissue cuff of the STA then is sewn to the edge of the dura using 5-0 suture in a running fashion. Over time, angiogenesis results in the formation of small arterial vessels to the brain (Fig. 5). Advocates of EDAS maintain that this procedure does not involve temporary occlusion of an MCA branch and is technically easier to perform. In addition, EDAS can be used in cases where there is not a suitable donor or recipient vessel, and it can also be used in conjunction with a STA-MCA bypass (see Fig. 2C). Matsushima and colleagues reported their results using EDAS to treat moyamoya disease in 38 pediatric moyamoya patients (70 hemispheres). In their experience, revascularization of the brain was obtained in 100% of the cases, with many patients showing improvement in symptoms. Suzuki et al. reported a series of 21 young patients with moyamoya disease, showing that EDAS resulted in increases in hemispheric and cortical flow, particularly in patients with TIA symptoms. In this study, new cortical vessels were noted as early as 2 weeks after EDAS. Encephalomyosynangiosis (EMS). In encephalomyosynangiosis, portions of the temporalis muscle are sutured to surgical dural defects, thereby approximating the muscle to the surface of the brain. As with EDAS, a frontotemporal craniotomy is performed, and the arachnoid layer is opened over as much of the brain as possible. The dural edges are secured to the edge of the temporalis muscle using 5-0 suture. Neovascularization occurs from the muscle tissue to the adjacent brain to supplement blood flow. Like EDAS, the EMS is easier to perform than the STA-MCA bypass, can be performed without identifying a recipient vessel, and can be combined with the STA-MCA bypass. However, this procedure has been associated with an increased risk of seizures. Several series have shown that EMS improves the clinical condition of patients as well as angiographic filling of the MCA vessels in 70% to 80% of cases. Omental Transposition. Omental transposition (see Figs. 4F and 4G) takes advantage of the large size and plasticity of the omentum to cover extensive areas of ischemic cortex, including entire hemispheres. The omentum can be used either as transposition flap based on gastroepiploic vessels or as a transplanted vascularized free flap. In either case, the omentum is harvested through a midline abdominal laparotomy. For transplantation procedures, the omentum is dissected out, and the gastroepiploic vascular pedicle is preserved, careful isolating the gastroepiploic artery and vein. The omentum is detached at its vascular pedicle immediately before transplanting it, to preserve perfusion as long as possible. The graft is kept moist, and the gastroepiploic artery and vein are transected and flushed with heparinized saline. The STA is dissected as described earlier, but for omental transplantation, the superficial temporal vein (STY) also must be dissected. A craniotomy is performed over the region to be revascularized, and an end-to-end or end-to-side anastomosis is performed between the exposed donor STA and the gastroepiploic artery, as well as between the STY and gastroepiploic vein, using 10-0 monofilament suture. The arterial anastomosis usually is performed first, with a temporary vascular clip placed on the artery during the venous anastomosis. Following this, the omentum is spread over the cortical surface and under the edges of the exposed dura. The graft is then sutured to the dural edges, and the bone flap is replaced with care taken not to compromise the vascular pedicle. Transposition of the omentum involves lengthening the omentum through dissection to reach the cranium while remaining attached to a vascular pedicle, with the possible advantage of preserving lymphatic drainage and avoiding the additional vascular anastomosis. The omentum is left attached to the dominant gastroepiploic pedicle, and lengthening of the omentum is carried out by dividing the omentum with L-shaped incisions. A subcutaneous tunnel large enough to accommodate the graft is developed along the chest wall, neck, and scalp, passing posterior to the ear. The pedicle must not be under tension or be twisted in any way as it is passed up through the tunnel; incisions often are made in the skin along the path of the tunnel to facilitate passage of the graft. Once at the cranium, the omentum can be placed over the surface of the brain as described earlier.
Postoperative Management The patient is transported to the intensive care unit for overnight observation after the surgical procedure is completed. Blood pressure should be monitored for the first 24 hours, because hypertension may cause bleeding from the anastomosis site, and excessive hypotension may result in graft thrombosis and occlusion. Coagulation studies should be checked and corrected if abnormal. Any changes in neurologic examination should be evaluated with emergent CT; we have had patients with omental transposition who developed mass effect as a result of the relative thickness of the omentum. Graft patency can be grossly assessed through palpation of the STA or the use of Doppler ultrasound. Angiography is reserved for cases in which there is a high index of suspicion of graft occlusion. We routinely perform cerebral angiograms at 2 to 6 months post-bypass to evaluate long-term graft patency, to document extent of the cerebral cortex filling from the graft, and to assess regression of moyamoya vessels. Xenon CT scans or other CBF studies are performed at the same time to assess improvements in blood flow. Complications There are several potential complications of STA-MCA bypass procedures. Ischemic changes usually are due to compression of the donor vessel by the scalp closure, graft occlusion or stenosis at the anastomosis site, or emboli. Doppler and angiography studies generally identify the cause. As mentioned earlier, hypertension may result in leak at the anastomosis or hemorrhage. Hypertension may cause cerebral edema and dysautoregulation or, occasionally, hemorrhage, especially with the higher flow external carotid to MCA saphenous vein grafts. Additionally, for STA-MCA bypasses, EDAs, and EMS, a non-water-tight dural closure is necessary to avoid any compression of the donor vessel, and this occasionally results in CSF leaks, pseudomeningoceles, and subdural hygromas. Finally, as with other craniotomies, systemic surgical complications include myocardial infarction, pneumonia, deep venous thrombosis, and pulmonary emboli. Complications from omental transposition include many of these adverse events, but also include the possibility of mass effect from the omentum itself. Additionally, the abdominal laparotomy required to harvest the omentum may result in an ileus, bowel perforations, and peritonitis. Outcome Surgical treatment of moyamoya disease using various revascularization techniques has been shown to be safe and effective in reducing ischemic events, in both adults and children. Clinically, revascularization in moyamoya patients has shown to be beneficial in reducing transient ischemic attacks, and, although the evidence is less conclusive, some studies suggest benefit for reducing hemorrhagic events. Post-revascularization angiography and MRI studies often reveal a reduction in moyamoya vessels that closely parallels improvement in symptoms. Additionally, reports in the literature that revascularization techniques result in improvement of cerebral blood flow studies, in both adult and pediatric moyamoya patients, are increasing in number.
©2003-2018
Web Vision Enterprises LLC.
All rights reserved. All information on this site is protected by international
Adamo HP, Kassell NF, WlSOff HS, Drake CG: Intracranial saccular aneurysm and
moyamoya disease. Stroke 10:174, 1979
|